Medical Devices Design Control
The Medical Device Life Cycle
When introducing a medical device to the market is important to follow standard guidelines so any person supervising our project can easily understand our job, including ourselves in the future.Depending on the scope of your organization or the particular project you are working on, standard operation procedures (SOP) should put in place in order to guarantee you did things good before first customer shipment. The SOP should define how design and development should be carried out.
The process is the same for all companies aiming to put in the market an effective, safe, reliable product is shown below as a Six Phases Life Cycle: Medical Device concept phase, Design concept phase, Medical Device design phase, Verification and Validation design phase, Production phase and End-of-life phase.
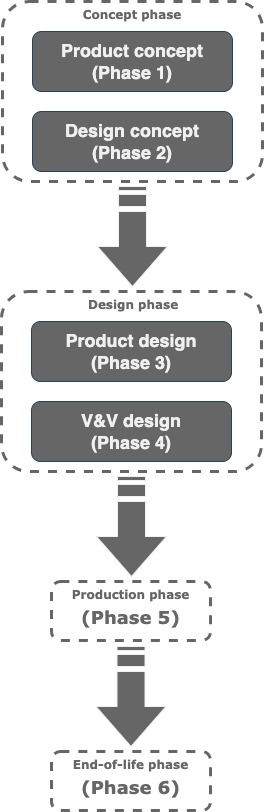
In particular, when designing a medical device, both FDA and MDR expect we have a Design SOP describing how we plan to control the design of a Medical Device from its concept up to production release.
Marketing a Medical Device in EU and MDR requirements
EU requirements are listed in the General Safety and Performance Requirements or GSPR included in Annex I of the Medical Device Regulation (MDR).To implement the GSPR we have the harmonized standards: if you meet the requirements of the STANDARDS you’ve normally fulfilled the corresponding GSPR.
Marketing Medical Devices in US and FDA regulations
For the US market there are no essential requirements or GSPR.
The FDA will expect the manufacturer to comply with the Quality System (QS) regulation (21 CFR Part 820) or QSR by implementig the requirements under the FDA Recognized Consensus Standards.
FDA has the authority to cover DESIGN CONTROL in their inspections.
The Pre-Market Notification Process (510k) and the Pre-Market Approval Application (PMAA) are the regulatory
pahtways for device approval.
For all but the lowest-risk medical devices, the FDA requires proof that you have done just that. The first step in meeting the FDA’s requirements is to develop your medical device under Design Controls.
The FDA guidance Design Control Guidance for Medical Device Manufacturers provides an understanding of what is meant by CONTROL in the conext of the requirements.
The Design Process under Control
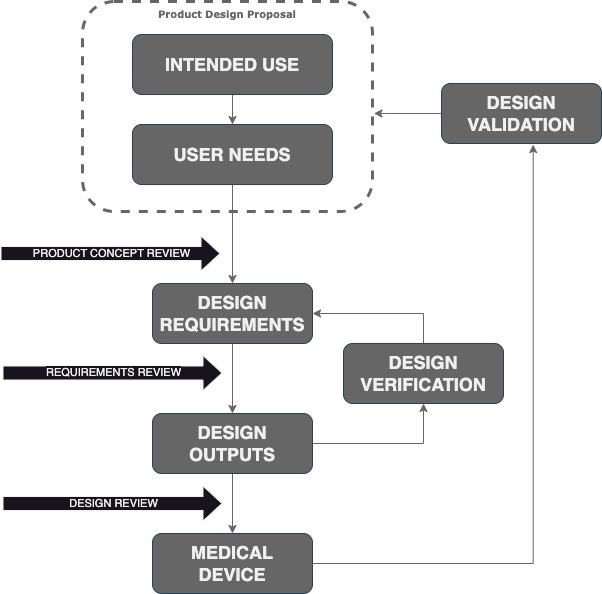
Intended Use Who will use the product? On whom, for what, when and where?
User Needs Identify the problem to solve. For example, the device should be autoclavable.
Design Inputs Finding the engineering design requirements that bring a solution to the problem. For example, the components should resist moist heat and high pressure.
Design Outputs At the end of the Medical Device design phase, there will be a complete product documentation as part of the design outputs: schematics, theory of operation, outline drawing, bill of materials (BOM), software, assembly, and mechanical drawings. There also be working prototypes suitable for design verification and validation (V&V)
Design Verification Verify that the design outputs meet the design requirements. This is different from design validation.
Design Validation Give evidence that the Medical Device, meets the Intended Use and the User Needs. Production units or equivalent can be used.
Risk Management Plan
As part of the regulatory requirements, the manufacturer shall conduct a risk management
process in accordance with the guidelines of ISO 14971.
The aim of this process is to provide a
comprehensive overview of the risk management activities and findings along the product life phases (see The Medical Device Life Cycle at the beginning) It includes:
- A Risk Management Plan
- A Risk Management Report
 Medical DevOps en Español
Medical DevOps en Español





