Control del Diseño de Equipos Medicos
Ciclo de Producto de un Dispositivo Medico
A la hora de poner un equipo medico en el mercado es importante hacerlo con todas las garantias que podamos ofrecer de manera que los profesionales y los pacientes puedan estar seguros de nuestro trabajo, incluidos nosotros mismos cuando tengamos que revisar el proyecto el dia de mañana.
Si bien el alcance de nuestro proyecto de diseño depende del tipo de producto en el que estemos trabajando, el procedimiento standard de como se lleva a cabo (SOP de Diseño o Procedimiento Standard de Diseño) debe de ser siempre el mismo y sus pasos definidos de manera clara en el Manual de Calidad de nuestra organizacion.
El procedimiento debe ser similar para cualquier organizacion que quiera introducir en el mercado un dispositivo medico efectivo, seguro y duradero. En el diagrama de abajo se muestran las 6 fases del Cicle de Vida de Producto de un equipo medico: concepto, diseño de concepto, diseño, verificacion, validacion, produccion y retirada-
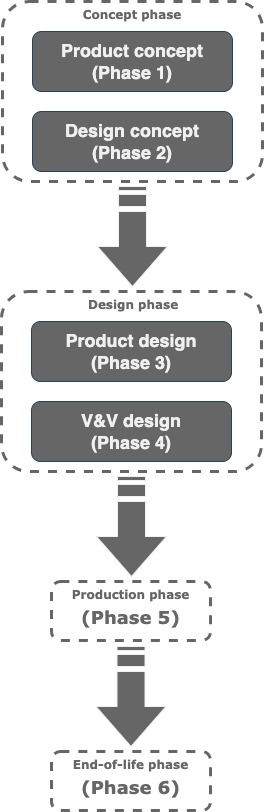
In particular, when designing a medical device, both FDA and MDR expect we have a Design SOP describing how we plan to control the design of a Medical Device from its concept up to production release.
Lanzamiento de Dispositivos Medicos en la UE. Requisitos de la MDR.
EU requirements are listed in the General Safety and Performance Requirements or GSPR included in Annex I of the Medical Device Regulation (MDR).
To implement the GSPR we have the harmonized standards: if you meet the requirements of the STANDARDS you’ve normally fulfilled the corresponding GSPR.
Puesta En Mercado USA Equipos Medicos. Normativa de la FDA.
En el mercado USA no existen requisitos esenciales o GSPR.
The FDA will expect the manufacturer to comply with the Quality System (QS) regulation (21 CFR Part 820) or QSR by implementig the requirements under the FDA Recognized Consensus Standards.
FDA has the authority to cover DESIGN CONTROL in their inspections.
The Pre-Market Notification Process (510k) and the Pre-Market Approval Application (PMAA) are the regulatory
pahtways for device approval.
For all but the lowest-risk medical devices, the FDA requires proof that you have done just that. The first step in meeting the FDA’s requirements is to develop your medical device under Design Controls.
The FDA guidance Design Control Guidance for Medical Device Manufacturers provides an understanding of what is meant by CONTROL in the conext of the requirements.
The Design Process under Control
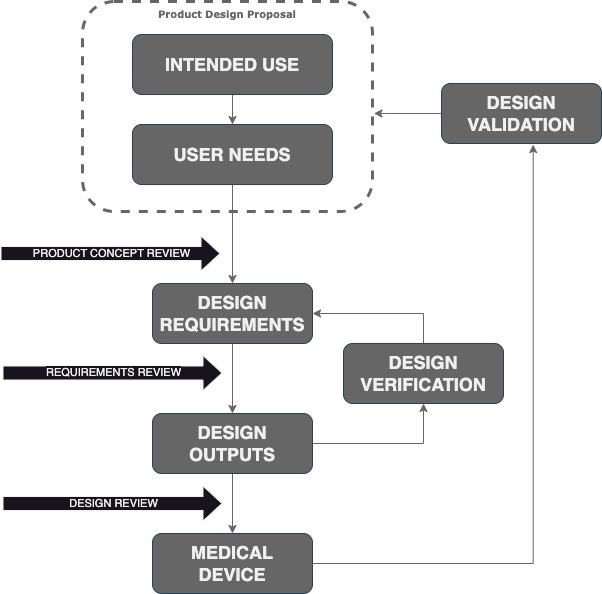
Intended Use Who will use the product? On whom, for what, when and where?
User Needs Identify the problem to solve. For example, the device should be autoclavable.
Design Inputs Finding the engineering design requirements that bring a solution to the problem. For example, the components should resist moist heat and high pressure.
Design Outputs At the end of the Medical Device design phase, there will be a complete product documentation as part of the design outputs: schematics, theory of operation, outline drawing, bill of materials (BOM), software, assembly, and mechanical drawings. There also be working prototypes suitable for design verification and validation (V&V)
Design Verification Verify that the design outputs meet the design requirements. This is different from design validation.
Design Validation Give evidence that the Medical Device, meets the Intended Use and the User Needs. Production units or equivalent can be used.








